By Paul Share, senior product development scientist, printing and packaging, BASF Corporation
Background

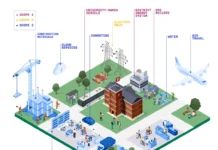
Several critical issues drive technological developments in UV/EB resin technology today, particularly within the fast-growing area of food packaging1. These are summarized in Figure 1.
High functionality resins are often used to address the need for higher cure speeds to meet the requirements for higher flexographic press speeds and corresponding improved efficiency and economics. Higher functionality often results in shrinkage, loss of flexibility and poor adhesion to film2. Lower oligomeric resin viscosity is preferred to the use of low functionality, low molecular weight monomers to reduce the total migration of a formulation. The combination of high functionality with high molecular weight in a resin can result in a reduced probability of migration, but it also can result in high viscosity, requiring the use of additional monomers to achieve a desired formulation viscosity.

The relationships between cure energy, viscosity and molecular weight for a wide range of UV monomers and oligomers are shown in Figure 2.3
Epoxy acrylates, ethoxylates, propoxylates, urethanes, polyesters and amine modified acrylates are included. Although monomer diluents are present in some of the resin systems, there is a clear trend of increasing viscosity and cure speed with increasing molecular weight. It also is apparent that the lowest viscosity and lowest molecular weight resins have some of the slowest cure speeds. Other systems, which are blends of inert resins with monomers, have the expected high molecular weight and high viscosity combined with low curing speed.
The viscosity/MW trend is consistent with the Mark-Houwink equation, which describes the relationship between the intrinsic viscosity η and the viscosity averaged molecular weight Mv.4
K and a depend on the specific polymer and conditions. The slope of log η vs log Mv will depend on the details of the resin chemistry, but there is a general proportionality. For linear polymers, a is typically 0.5 to 0.8. The viscosities are usually measured in solvent, and the specific solute-solvent interactions can influence the value of a. The values in Figure 2 were all measured neat. There is a noted exception to the typical a values, which results from a nonlinear or hyperbranched structure.5

As shown schematically in Figure 3, the relationship between molecular weight and viscosity also is highly dependent on molecular structure. Dendrimers, which are very ordered with regard to molecular weight and geometry, have higher viscosity at low molecular weights, which then decrease with increasing molecular weight due to reduced entanglement resulting from their spherical shape. In addition, at low molecular weights the structure of the dendrimers is different, with higher fractions that do not contain the core molecule.7
Hyperbranched structures are intermediate between linear and dendrimeric, possessing less order than dendrimers but a lower probability of entanglement than linear polymers due to a more compact configuration. This concept is summarized pictorially in Figure 4.

In addition to factor of molecular shape, the specific chemical composition of the polymer plays a critical role. In 100 percent UV formulations, just as in solution, the viscosity will be affected by hydrogen bonding, the molecular dipole moment and the Hildebrand solubility parameter.8 Therefore, in addition to the dendrimeric/hyperbranched/linear structural effects, the specific building blocks used in the polymer design can determine the viscosity and reactivity.
The application of chemistries with dendritic structures in UV/EB applications has been investigated for some time.9,10,11 The challenge for low-migration packaging applications is that high levels of polyether diluents can be necessary to achieve fluidity at ambient temperature. Even though the viscosity of the dendrimeric acrylate is much lower than that of the corresponding linear acrylate of comparable Mw, the viscosity still is quite high, and the dendrimeric backbone often is a solid at room temperature7. This is presumably due to the polarity of the polyester backbone, which comprises the dendrimeric polyol as well as the presence of hydrogen bonding.
In addition, the increase in functionality also leads to a decrease in the rate of double-bond conversion as a result of the Trommsdorff effect.12 If gelation of the reaction mixture occurs prior to consumption of the double bonds, then there are no property advantages, such as chemical resistance, to result from the added functionality. In a study of acrylate functionalization of a 19-functional dendrimeric polyol, the highest conversion rate was found to occur at an acrylate functionalization of 5.7
Experimental
Raw materials were weighed and blended to produce the formulations reported. Coatings were applied with a wire rod at a film build of 1 mil on Leneta® cards. Ink dispersions were premixed in a high-speed mixer, and then dispersed on a high-speed media mill to a grind of 5 microns or less. Inks were applied to untreated OPP film with a flexo proofer at a film build of 1.8 microns. All samples were cured with a D bulb under ambient conditions. Curing energy was the lowest energy at which a mar-free coating or ink was obtained. The UV exposure was measured with an EIT™ radiometer model PP2000. Ink adhesion was determined using Scotch™ Brand 610 tape. The relative amount of ink remaining after tape removal was recorded and reported as percent adhesion.
Results and discussion

Low viscosity monomers often are necessary to achieve the rheological properties necessary for flexographic applications, but they bring with them challenges relating to migration, often due to low molecular weight or low functionality. The higher reactivity resins often bring challenges relating to viscosity, illustrated in Figure 1, as the highest reactivity resins fall on the higher end of both the molecular weight and viscosity scales. The objective is to achieve a balance between reactivity and diluency.
To better understand this effect, a study was made of the viscosity and cure energy of a number of resin compositions. One oligomeric system was selected from the broad categories of epoxy, polyester, urethane and polyether acrylates. Each oligomer then was blended with DPHA, TMPTA, TPGDA and HRLV (a novel high-reactivity, low-viscosity resin). The results of this study are shown in Figure 5.

In all cases, the order of viscosity was determined to be TPGDA TMPTA > TPGDA. These resins are summarized in Table 1.
In Figure 2, the data point corresponding to HRLV has a molecular weight of 1200 amu and a viscosity of 500 mPas. Based on the earlier analysis of the Mark-Houwink equation, this resin would be expected to assume a more spherical configuration, which may also be a factor in the higher cure speed of blends that contain HRLV. Despite the differences in polarity and intermolecular interactions within the classes of oligomeric resins in Figure 2, the variation in viscosity and cure speed by addition of the diluent resins follows a consistent trend.

Dispersing additives with spherical structures have been shown to be effective in pigment stabilization in reactive systems14. If the HRLV resin assumes a spherical configuration, then it also may have properties in pigmented systems that are different from those of conventional linear polymers. To evaluate this idea, flexo inks were prepared from cyan, magenta, yellow and black pigments and tested for cure, color density and adhesion. The test formulations are shown in Table 2, and the results of this study are shown in Figures 6 and 7.


Although there are variations across pigments, some clear trends are shown. The two polyester oligomers yield inks with similar color densities and cure speeds. The HRLV resin, presumably with a more spherical rather than linear structure, provides color densities and cure speeds that are superior to the linear polyesters, with the exception of the yellow ink, where the performance is the same. All formulations utilized the same HMWD15, and it may be that that the three-component system of oligomer, pigment and HMWD could be further optimized in combination with specific pigment chemistries. What is clear, however, is that through the controlled use of the three-dimensional resin structure, differentiation in ink cure speed and color density can be obtained.

With sufficient photoinitiator loading, a leveling effect can occur, masking differences in formulation reactivities. This comes at a higher overall formulation cost, since the photoinitiator level is not optimized. To determine whether this played a role in the flexo ink performance, the photoinitiator levels in the flexo ink formulations were reduced by 25 percent, and the cure energies were reevaluated. The results of this experiment are shown in Figure 8. At the lower photoinitiator level, the differences in cure energy between the HRLV inks and the two polyester inks are apparent. It would require the addition of almost 25 percent more photoinitiator to the polyester formulations to approach the cure energy of the HRLV inks.
The idealized nonlinear resin structure has low free volume, and therefore the density increase and volume reduction that occur during the crosslinking process will be smaller than for an idealized linear resin. The smaller change in density between the liquid and cured ink also can be accompanied by an increase in adhesion to films, as compared to that of a linear resin, due to the reduced shrinkage and stress. Although a number of variables, including surface energy, affect adhesion, shrinkage is a significant factor. The adhesion properties of the ink systems are shown in Figure 9.

There are clear differences in the adhesion properties between ink systems shown in Figure 9 which do not parallel the trends that are typically seen in the cure of linear polymers. A slower curing system might be expected to have higher film adhesion due to reduced film stress, and a faster curing system would be expected to have lower film adhesion to due increased film stress. These results show that the structure and properties of the HRLV not only increase cure speed but also increase OPP film adhesion compared to linear polyester oligomers.
Conclusions
The viscosity and molecular weight relationship of commercial UV resins has been examined through the lens of the Mark-Houwink equation to better understand the effect of molecular shape on coating and ink performance properties. An HRLV resin, which was predicted to have a nonlinear configuration on this basis, was evaluated for diluency, color density, cure energy and film adhesion properties in flexo ink formulations. In comparison to conventional polyester resins at the same formulation levels, flexo ink formulations containing the HRLV resin provided lower cure energy, higher color density and better adhesion to untreated OPP film. In terms of the structure/property relations discussed, HRLV has the high reactivity that would be expected for a highly functional resin but does not have the high viscosity which is associated with highly functional linear resins. HRLV has the superior adhesion associated with the low free volume spherical resin structure but does not have the high viscosity and need for polyether diluents that can be required for dendrimeric structures. In addition to low viscosity, HRLV has the molecular weight and functionality that make it suitable for low-migration packaging applications.
Acknowledgements
The author would like to thank Dr. Sebastian Berger, Dr. David Tuerp, Stephen Godlew, and William Merritt for their contributions to this work.
References
- “The Future of Package Printing to 2015,” Pira International Ltd.
- Chiang, T.H.; Hsieh, T.E., Intl. Journal of Adhesion and Adhesives, 26, 2006, p. 520.
- Data obtained from BASF Laromer™ Product Literature.
- “Introduction to Polymers,” R. J. Young and P.A. Lovell, 1991, pp 196-198.
- Gorodetskaya, I.A.; Choi, T.; Grubbs, R.L., J. Am. Chem. Soc. 129, 42, 2007, p.12672.
- Jikei, M,; Kakimoto, M. Prog. Polym. Sci. 26, 2001, p. 1233.
- Zagar, E.; Zigon, M. Prog. Polym. Sci. 36, 2011, p. 53.
- Miller-Chou, B.A. ; Koening, J.L. Prog. Polym. Sci. 28, 2003, p. 1223.
- Klang, J. Radtech Technical Proceedings, 2006.
- Sangermano, M., Radtech Report, 3, 2012.
- James, D.; Bernquist, H.; Appleqvist, P.; Sandell, P.; Sörenson, K., RadTech 2006 Technical Proceedings.
- Tulig, T.; Tirrell, M. Macromolecules 1982, 15, 459.
- Formulations contain a blend of 30% diluent with 66% oligomer and 4% Irgacure™ 500. Numerical values over the polyether data represent viscosity and are added for clarity.
- Rudolfi, A.; Krohnen, M.; Piestert, F.; Mößmer, S., European Coatings Journal, 11, 2013, p. 22.
- HMWD (high molecular weight dispersant).
- Inks applied to OPP at 1.8µ film thickness and formulated to 1000 mPas viscosity. Pigment levels of cyan, magenta, yellow and black inks are 15.0%, 12.3%, 10%, and 12.5%, respectively.
- Samples cured with D bulb at 1.8µ film thickness.
Leneta® is a trademark of Leneta Company, Inc.
EIT® is a trademark of Electronic Instrumentation and Technology LLC.
Scotch™ Brand 610 tape is a registered trademark of 3M.
IRGACURE® is trademark of BASF SE.
® Registered trademarks of BASF Group.
© 2016 BASF Corporation
Dr. Paul Share is senior product development scientist, Printing & Packaging, for BASF. Share received his B.S. in Chemistry at the University of Chicago and his Ph.D. in Organic Chemistry at the University of California Berkeley. He then did postdoctoral work at the Max Planck Institut for Quantenoptik in Garching, Germany and at the University of Pennsylvania, working in the area of organic photochemistry and photophysics. With previous positions at Henkel and Valspar, Share joined BASF in 2011, where he is responsible for global UV/EB resin development for printing and packaging applications.