By Syunyua Horii, Chief, R&D Division, Osaka Organic Chemical Industry Ltd.
Photocurable compositions containing photoinitiators and polymerizable monomeric components are widely used in additive manufacturing, paints, inks, adhesives, sealants, insulating films, protective coatings and films. Since 2005, when it was confirmed that certain components from UV-curable inks migrated into food products, there has been growing concern about the safety of UV-curable printing. This is less of a concern with water-based and EB-curing printing systems, but there still are concerns about migration of surfactants, and the cost can be too high. For this reason, there is growing interest in initiators that can be fixed as polymerization components.
With conventional photoinitiators, their toxicity can be a problem, particularly in food packaging applications. In addition, conventional photocurable compositions have the problem of migration of the photoinitiators themselves and their degradation products from the cured products. Azusa Yogo et al. reports that migration can be reduced by increasing the average molecular weight of the photoinitiators above 320 Dalton. 1 PI-1 (hereafter, Photoinitiator A) is a newly developed monomer that contains a polymerizable moiety and a photoinitiator moiety, allowing it to copolymerize with the polymer to reduce migration. As it is not a cleavage-type system, it is expected to reduce the amount of low-molecular-weight photolytic-decomposed products.
Photoinitiator A has a hydrogen abstraction moiety that differs from conventional cleavage-type photoinitiators. UV excites the hydrogen abstraction moiety, and it initiates polymerization by coexisting hydrogen-donating monomers. The use of hydrogen abstraction photoinitiators (Type II) is limited because they have a much slower polymerization rate than cleavage-type photoinitiators (Type I). Therefore, the author’s company investigated the influence of the hydrogen donor structure.
Experiment
Confirmation of photo-polymerizability of Photoinitiator A
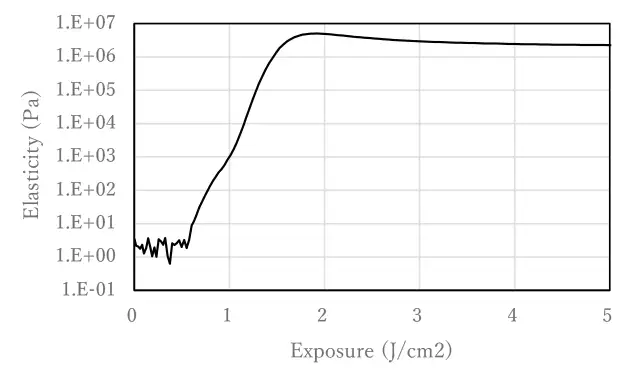
10 mol% Photoinitiator A and 10 mol% 2-hydroxyethyl acrylate (HEA), an acrylate with hydrogen-donating moieties, were added to IBOA (isobornyl acrylate) and mixed. Viscoelasticity was measured using a rheometer under UV irradiation with a mercury xenon lamp.
Effect of structure of hydrogen-donating monomers
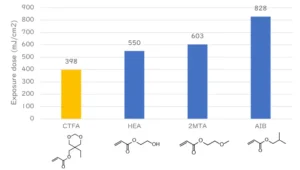
10 mol% Photoinitiator A and 10 mol% cyclic trimethylolpropane formal acrylate (CTFA), 2-hydroxyethyl acrylate (HEA), 2-methoxyethyl acrylate (2MTA) and isobutyl acrylate (AIB) were added to IBOA and mixed. Viscoelasticity then was measured using a rheometer under UV irradiation with a mercury xenon lamp. The irradiation dose at which the elastic modulus began to increase was defined as the polymerization-initiating irradiation dose.
CTFA was a highly purified CTFA manufactured by the author’s company to eliminate the effect of any multifunctional ingredient and the recent toxicity problem of TMPTA.
Comparison with cleavage-type initiator
0.1 wt% or 1 wt% Photoinitiator A was added and mixed with CTFA. Viscoelasticity was measured using a rheometer under UV irradiation with a mercury xenon lamp. For comparison, 0.1 wt% of 2-hydroxy-2-methyl-1-phenylpropanone (Irgacure 1173), a cleavage-type initiator (Type I), was added to CTFA and mixed. Viscoelasticity was measured using a rheometer under UV irradiation with a mercury xenon lamp.
Migration test of initiators
To test initiator migration, 5 mol% pentaerythritol triacrylate (PETA) as the base monomer, 75 mol% CTFA as the diluent and 20 mol% Photoinitiator A as the photoinitiator or diphenyl 2,4,6-trimethylbenzoyl phosphine oxide (TPO) for comparison were mixed. The UV-curable composition was applied to an aluminum plate to form a 20 µm film using a bar coater, and then UV-irradiated with 3 J/cm2 using a high-pressure mercury vapor lamp to produce a cured film. The cured film was scraped off, immersed in acetonitrile/water = 90/10 (vol/vol) for 30 minutes, and measured by liquid chromatography to perform a migration test. The migration rate (%) was defined as migration weight / added weight.
Result and Discussion
Confirmation of photopolymerizability
As shown in Figure 1, the viscoelasticity of the photocurable composition increases with UV irradiation, indicating polymerization. The use of hydrogen-abstracting Photoinitiator A shows that polymerization proceeds by UV irradiation without the use of a cleavage-type photoinitiator.
Effects of structure of hydrogen-donating monomers
CTFA, which has a methylenedioxy group, has been shown to require less irradiation to initiate polymerization and has a higher initiation efficiency than the other hydroxy or ether monomers (Figure 2).
Hydrogen abstraction occurs from the weak C-H bond. The author’s company believes that this is because the methylenedioxy group, which is sandwiched between electron-rich oxygen, produces more stable radicals and is more likely to generate carbon radicals, the active radicals involved in polymerization, than compounds such as simple hydroxyl groups and ethers. Again, the CTFA used is highly purified and does not contain impurities such as trimethylolpropane triacrylate (TMPTA).
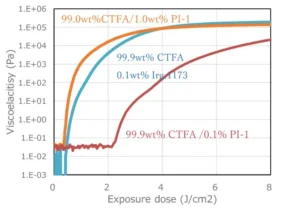
Comparison of hydrogen-abstraction (Type II) and cleavage-type initiators
(Type I)
The combination of CTFA and Photoinitiator A, which had a relatively high initiation efficiency, required approximately 10 times the amount added to achieve the same effect compared to Irgacure 1173 (Figure 3). The cleavage-type initiator is excited by UV irradiation and cleaves intramolecularly to form radicals, whereas the hydrogen abstraction type initiator has a rate-determining rate of hydrogen abstraction from the hydrogen donor after excitation, resulting in a comparatively low polymerization initiation efficiency.
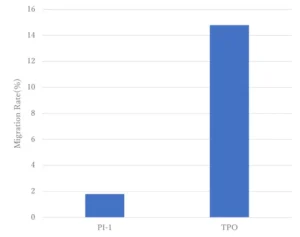
Migration test of initiators
Figure 4 shows the results of the initiator migration test. It confirms that the migration is suppressed compared to that of TPO used as a conventional photoinitiator. This indicates that the Photoinitiator A has been covalently bound into the polymer network of the cured film and hence the potential for migration should be minimized.
At present, it is not yet compatible with UV LED because the polymerization efficiency of Photoinitiator A is lower than that of conventional cleavage-type photoinitiators. In the future, studies will continue to make it compatible with UV LED.
Conclusion
The author’s company reported a new low-migration photoinitiator. It may be applicable to various kinds of applications, such as additive manufacturing, paints, inks, adhesives, sealants, insulating films, protective coatings and films.
Reference
- Azusa Yogo et al. ACTIVE ENERGY RAY-CURABLE OFFSET INK COMPOSITION. WO2014156812A1
Suynya Horii is chief of the R&D division of Osaka Organic Chemical Industry Ltd. Horii holds Master of Division of Applied Chemistry from Osaka Prefecture University, granted in 2012. Horii joined with Osaka Organic Chemical Industry in 2012 as a researcher and has been focusing on Chemical Engineering for a long time. For more information, email syunya_horii@ooc.co.jp.