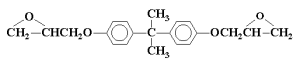
People new to UV/EB polymerization quickly learn that most of the oligomers used in energy-curable formulations have very high viscosities, often more than 1 x 106 centipoise (cP). A classic example of such a highly viscous material is an acrylate-functional epoxy oligomer based on the diglycidylether of bisphenol-A (DGEBA). Figures 1 and 2 depict the molecular structure of DGEBA and its diacrylate ester, respectively. The simple process of reacting DGEBA with acrylic acid has produced an increase in the viscosity of the material of nearly a thousand-fold!
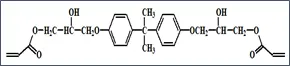
What is the primary reason for the very large viscosity increase experienced when DGEBA is made acrylate-functional? The answer involves the significant increase in the intermolecular attractive forces among the molecules following the acrylation step. DGEBA is a relatively low-polarity compound consisting primarily of a nonpolar aromatic hydrocarbon core structure along with two weakly polar ether linkages and two oxirane (epoxy) structures. The electronegativity difference between the oxygen atoms and the carbon atoms to which they are bonded creates relatively weak dipole-dipole attractions among the molecules. And, as with all atomic, ionic and molecular species, this compound also exhibits instantaneous dipole-induced dipole attractions or “London Dispersion Forces” (LDFs). These attractive forces account for DGEBA’s relatively high viscosity for a liquid species of around 12 000 cP, a viscosity that is approximately twelve-thousand times that of water!
The diacrylate ester of DGEBA contains the same nonpolar hydrocarbon core as its epoxide-functional starting material, but it also contains two quite polar acrylate end-groups. Additionally, and more importantly, the compound contains two very polar secondary alcohol groups (-OH). These -OH groups create very strong forces of attraction among the molecules – forces known as hydrogen-bonds or “H-bonds.” These H-bonds are the primary reason for the substantial increase observed in the viscosity.
The oligomer typically is the primary or foundational component of any UV/EB-polymerizable formulation and is selected by the formulator to match the principal properties needed in the end-use application. For example, if a particular conventional end use required an aliphatic polyurethane, then the UV/EB formulator would select an acrylate-functional aliphatic urethane oligomer as a starting point for formulating the product. In an ideal world, the oligomer might be used in neat form without dilution. However, its high viscosity is a problem for both manufacturers and formulators. This necessitates the addition of other components to reduce its viscosity.
In conventional coatings, inks, adhesives, etc., the viscosity of the oligomer or resin often is reduced with various organic solvents. However, in UV/EB polymerization processes, this is undesirable due to the environmental impact of volatile solvents and their flammability. Therefore, as is well understood, nonvolatile, nonflammable functional monomers that copolymerize with the oligomer during energy curing are used to reduce its viscosity to acceptable levels. These monomeric diluents are, thus, retained in the final product and are not evaporated. This creates a situation wherein the monomer selection process is complicated by the fact that the properties of the final end-use product are substantially affected by the presence of these monomers. If a simple, inexpensive method for reducing the effects of the H-bonding on the viscosity of the oligomer could be developed, perhaps the concentration of monomers necessary to get a workable product could be reduced significantly.
Approaches to Solving the Problem
Student researchers in the Center for Applied Polymer Science Research (CASPR) lab at the University of Houston-Downtown conducted experiments to find a relatively simple and inexpensive approach to reducing the viscosity of this oligomer without using monomers. This work began with an investigation into the claims of US Patent 4,687,8061. The patent, issued in 1987 to Interez, Inc. (now an allnex business unit), claimed that quite small amounts of an aqueous lithium bromide solution [LiBr (aq)] could substantially reduce the viscosity of the diacrylate ester of DGEBA. That work was predicated on the assumption that the lithium ion (Li+) should have the right size and charge density to interrupt the H-bonding. The primary formulations tested contained 98% by mass of the oligomer and 2% by mass of various mixtures of deionized water (DI H2O) and LiBr. The optimum ratio was found to be 1.85/0.15 DI H2O/LiBr. While the researchers found that LiBr (aq) did reduce the oligomer viscosity significantly, they also found that pure DI H2O was even more effective at reducing the viscosity than any of the LiBr (aq) solutions. Their results were reported in 2011 at the Photopolymerization Fundamentals Conference in Breckenridge, Colorado, and at the 2012 RadTech UV+EB Expo and Conference in Chicago2, 3.
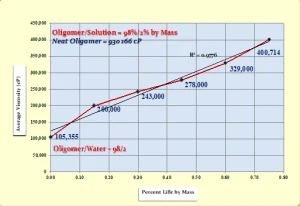
Figure 3 shows the effects of increasing the LiBr concentration on the viscosity of the oligomer/LiBr (aq) blend. It can be seen that the viscosity of the solution containing 0.15 % by mass LiBr was nearly 90% higher in viscosity than the formulation containing only DI H2O. Further, the viscosity continued to increase in essentially linear fashion as the LiBr concentration was increased. The hypothesis that the Li+ would interrupt the H-bonding appears to have been refuted. In fact, in a separate experiment, 2% by mass of LiBr was added to the neat oligomer without any DI H2O. This resulted in a highly viscous elastomeric liquid that stretched somewhat like a rubber band indicating that the Li+ was acting as an ionic cross-linker rather than interrupting the H-bonds! To test this, DI H2O was back added to the elastomeric mixture and the viscosity was reduced accordingly. Hydration of the Li+ ions apparently caused a breakup of the ionic crosslinks.
For the neat oligomer sample used in the experiment illustrated in Figure 3, simply blending in 2% DI H2O reduced its viscosity by about 89%. The researchers then sought to determine the limits of DI H2O’s compatibility with the oligomer. They found that DI H2O was completely miscible with the oligomer in all proportions up to 4% by mass. At 5%, a hazy heterogenous mixture was observed. It is interesting to note that if it is assumed that two molecules of H2O are needed to interrupt the H-bonds between two molecules of the oligomer, the mass ratio of water/oligomer is about 3.7% – close to the 4% observed in the experiment.
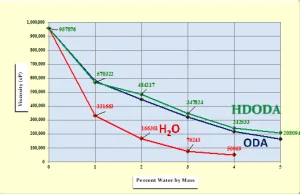
How does the diluency of DI H2O compare with acrylate-functional monomers? According to work presented at the Radcure ’86 Conference in Baltimore, Maryland4, octyl/decyl acrylate (ODA) and 1,6-hexanediol diacrylate (HDODA) were two of the very best monomers for reducing the viscosity of diacrylate-functional oligomer based on DGEBA. With this in mind, a comparison was made of the viscosity-reducing ability of DI H2O with that of ODA and HDODA. Figure 4 shows that DI H2O at a concentration of only 2% by mass gave a significantly lower viscosity than did either ODA or HDODA at 4%! Clearly, DI H2O is a very effective and inexpensive viscosity reducer for this oligomer.
Having demonstrated that DI H2O was a very effective diluent for this oligomer, a second phase of the investigation was initiated for the purpose of determining whether the presence of DI H2O would create formulating problems with relatively nonpolar monomers. That work will be discussed in the next edition of “Professor’s Corner.”
 Byron K. Christmas, Ph.D.
Byron K. Christmas, Ph.D.
Professor of Chemistry, Emeritus
University of Houston-Downtown
b4christmas@gmail.com
REFERENCES
- U. S. Patent 4,687,806, issued to Interez, Inc., William J. Morris (deceased), Richard R. Kemmerer, and Byron K. Christmas, August 18, 1987.
- Byron Christmas, “Reducing the Viscosity of an Epoxy-based Oligomer Using Minimal Amounts of Aqueous Electrolyte Solutions: Revisiting the Use of Water as a Diluent for UV-Polymerizable Oligomers”, Photopolymerization Fundamentals Conference 2011, Breckenridge, CO, June 27, 2011.
- Nameera Baig, Mau Truong, Tori Dumas, and Byron Christmas, “A Simple Process for Preparing Low Viscosity Diacrylate-Functional Epoxy Oligomers”, Proceedings, RadTech UV & EB Technology Expo & Conference, Chicago, IL, April 30-May 2, 2012.
- Byron Christmas and Ed Zey, “UV-Curable Monomer Comparisons, 1: Cured Film Properties of Oligomer/Monomer Blends”, Proceedings, Radcure ’86 Conference, Baltimore, MD, 1986.




