For any given technology, there are myths and misconceptions of how that technology works, how that technology can be implemented, and all the other little whats and whys and hows that surround using that technology for a particular application. Electron beam is no exception to this rule, and, as a technology that has less everyday familiarity than some, it certainly is prone to misinformation. Let’s tackle a few misconceptions.
When users switch to EB, the coating application method needs to change.
Beware the blanket statement. Switching from what technology? For what application? The origin of this myth likely stems from the fact that EB inks/coatings (just like UV, in this instance) most often are more viscous than solvent- or water-based inks/coatings. That said, EB inks/coatings span a pretty wide gamut of viscosities and are designed specifically for the printing/coating method for which they were intended – flexo coatings for flexo coaters, inkjet inks for inkjet printers, etc.
However, while there are many instances where switching to EB is straightforward, it isn’t always quite as easy as just pumping an EB coating into the coater. Because EB inks/coatings don’t have an evaporative fraction (i.e., wet coat weight = dry coat weight), a different anilox size may be needed, for example, if the user is used to using gravure coating with solvent- or water-based coatings. The higher viscosity of the EB formulations also can cause a difference in the transfer volume of an anilox; not to mention, it may require a different coat weight all together to achieve the gloss, scratch resistance and heat resistance properties the user is looking for. So, users should be aware that adjustments may be needed – but don’t think trading in a gravure coater for slot-die or anything too crazy will be needed.
EB processes require nitrogen because the beam is absorbed
by oxygen.
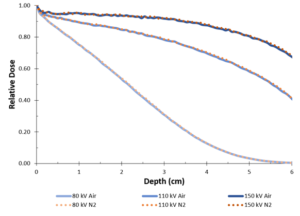
Not quite. Some energy is transferred to oxygen molecules in the air when an accelerated electron collides with those molecules through a process called inelastic scattering. ,sup>1 Yet, this process can occur with any atom or molecule, including nitrogen. The difference between oxygen and nitrogen is that oxygen reacts to form ozone when exposed to the beam and nitrogen is inert. To demonstrate that air does not absorb any additional electron beam energy than pure nitrogen, Monte Carlo simulations were run in the two different atmospheres at three different accelerating voltages (Figure 1).
The real reason that nitrogen is commonly used in EB processes is that free-radical chemistries, which are the predominant class of EB-curable inks, coatings and adhesives used in industry, are inhibited by oxygen. 2 This is a well-known phenomenon that is not unique to EB; however, other technologies, such as UV, have found other mitigation methods.
All EB processes need less than 200 ppm oxygen.
Ooh, what did I say about blanket statements being suspicious? This misconception has a good chunk of truth to it, as alluded to in the rebuttal to the previous myth, but that “all” part is the kicker. For free-radical, EB-curable inks, coatings and adhesives that are exposed to an atmosphere when being cured, a low concentration of oxygen is necessary to achieve acceptable levels of polymerization. The industrial standard for low concentrations of oxygen is 200 ppm oxygen or less. In reality, different formulations have different sensitivities to oxygen; some fare just fine at 500 ppm oxygen, while others would require 50 ppm oxygen to properly cure. The concentration of oxygen is moot, though, if the coating, ink or adhesive is not exposed to an atmosphere, such as in the cases of lamination, Cast and Cure™ or cold-foil transfer.
The other exceptions to the 200-ppm rule include the multitude of processes that don’t rely on free-radical driven wet chemistry, such as crosslinking or sterilization. These processes still can lose free radicals to oxygen inhibition but often with negligible consequences. Thus, these processes often are run in air, and the ozone that is created either is exhausted or captured and destructed per state/country regulations. u
References
- Woods, R.J., Pikaev, A.K., Applied Radiation Chemistry: Radiation Processing, 1994.
- Odian, G.G., Principles of Polymerization, 2004.
 Sage Schissel, Ph.D.
Sage Schissel, Ph.D.
Applications Specialist
PCT Ebeam and Integration LLC
sage.schissel@pciebi.com