In the first issue of Professor’s Corner, three objectives for the column were outlined. 1 The second objective for the column, “… is to provide a framework for the clarification of terminology used in the industry.” The third edition of the column began this process with the following:
“People entering the polymer technical workforce for the first time are immediately confronted with a wide range of terms and definitions, many of which are used interchangeably, and, of greater concern, many often are used incorrectly. While there is certainly no perfect vocabulary for the UV/EB professional, it may be useful to confront this linguistic issue directly.
Perhaps a good place to start is with the words ‘curable,’ ‘cured’ or ‘curing,’ as in ‘UV-curable,’ ‘energy-cured’ or ‘radiation curing.’ When a polymer is ‘cured,’ a linear or branched polymeric species is subjected to a subsequent chemical process wherein the macromolecules are linked together by covalent bonds. The terminology comes from traditional conventional thermal or air curing processes. However, this is not typically what happens with UV or EB ‘curing.’ Rather, in most cases, a mixture of non-polymeric oligomers and monomers of relatively low molecular mass are coated or printed onto a substrate and, subsequently, they are polymerized and crosslinked (cured) simultaneously. While this is not a critical linguistic issue, it is, perhaps, more accurate to suggest the terms ‘UV polymerization,’ ‘EB polymerization’ or ‘energy polymerization’ be used in technical papers and presentations to describe the process. Likewise, in speaking of the raw materials (oligomers and monomers) in a typical formulation, it is suggested that these be referred to as ‘UV-polymerizable’ or ‘EB-polymerizable’ materials. Certainly, these materials are being cured, but not in the conventional thermal-cure sense. Rather, they are non-polymeric materials that, when exposed to UV or EB sources of energy, polymerize and crosslink in situ.” 2
Continuing this process, let’s look at the terminology used for basic raw materials involved in energy polymerization. A typical UV/EB-polymerizable formulation consists of oligomers, monomers, photoinitiators and other additives.
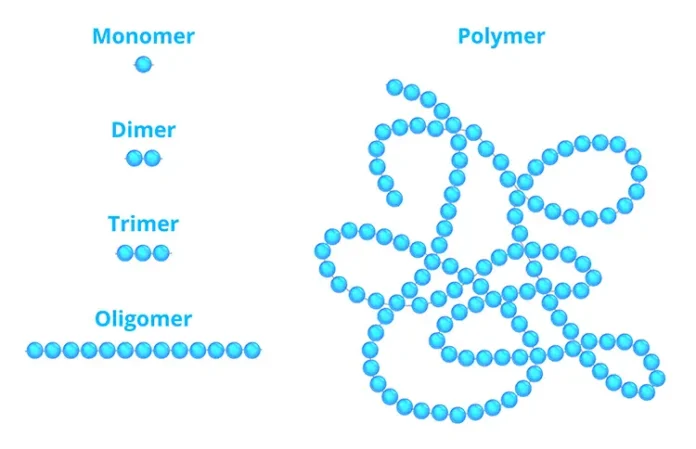
OLIGOMER
Monomer: A single chemical species capable of reacting to form very large “macromolecules”
Dimer: Two monomers linked together
Trimer: Three monomers linked together
Tetramer: Four monomers linked together
Etc.
Oligomer: Several or a few monomers linked together
These terms undoubtedly are familiar to every reader of this article. But the term oligomer potentially presents two linguistic issues:
- Many, if not most, key oligomers, such as the diglycidylether of bisphenol-A diacrylate (DGEBA-DA) – a common “epoxy acrylate” – are, in fact, monomers; they contain a single reactive molecule or “mer.”
- They often are referred to as “resins.”
Both issues arise from the fact that the oligomers used in UV/EB polymerization processes typically have relatively high viscosities, substantially higher than that of the monomers used in formulations. The simplest (lowest molecular mass) DGEBA-DA, for example, has reported viscosity values ranging from about 9.5 x 105 cps to about 1.2 x 106 cps – several orders of magnitude higher than those of typical monomers.
Monomer vs. Oligomer
In spite of this seeming contradiction of terms, there is a clear need to differentiate the oligomer(s) in a formulation from the monomers. Not only does it have a much higher viscosity than the monomers, but it also is the primary component in the formulation with respect to the specific properties desired in the final product. Therefore, it seems appropriate to continue the broad use of the term oligomer for these key formulation components.
Resin vs. Oligomer
The Merriam-Webster dictionary defines a resin as: “Any of various solid or semisolid amorphous fusible flammable natural organic substances that are usually transparent or translucent and yellowish to brown, are formed especially in plant secretions, … and are used chiefly in varnishes, printing inks, plastics, and sizes and in medicine.” 3
Thus, the term comes originally from natural, plant-based materials, such as tree sap or similar substances. These, of course, are high-viscosity, sticky, gooey materials similar in properties to some UV/EB-polymerizable oligomers.
The term also has been subsequently adopted for many synthetic polymer-based materials, as is indicated in this second definition by Merriam-Webster: “Any of a large class of synthetic products that have some of the physical properties of natural resins but are different chemically and are used chiefly in plastics.” 3
So, most oligomers used in UV/EB polymerization have resin-like properties, making the term resin seem appropriate. However, it is an archaic term used for a very large number of different polymer-based technologies that significantly pre-date the commercial development of photopolymerization processes. Additionally, the term resin often is used for a completely formulated product, while the term oligomer specifically refers to a single component in an energy-polymerizable formulation. Thus, the term oligomer is more specific and is identified with a more sophisticated and advanced technology.
Photoinitator
Perhaps the confusing use of various terms for photoinitiators represents an even greater need for clarification. The following terms often are used interchangeably, though they are not really synonyms in many cases: photoinitiator, photosensitizer and coinitiator.
The photoinitiation process generally proceeds by one of two different mechanisms:
- Norrish Type I – Photocleavage
- Norrish Type II – Photoabstraction
Photocleavage-type photoinitiators absorb UV or visible light energy and are, thus, energized to an excited state. From this state of excitation, they then dissipate the excess energy through direct homolytic cleavage of a single bond to form two distinct free radicals. The term photoinitiator is appropriate in this case since a single molecule is involved in both the energy absorption step and the free radical generation step. Common examples of this type of photoinitiator system include 1-hydroxycyclohexyl phenyl ketone (Irgacure®184) and 2-hydroxy-2-methyl-1-phenyl-propan-1-one (Darocur®1173).
Photoabstraction-type photoinitiator systems, on the other hand, typically involve two different molecules: one that absorbs the energy and is known as the photosensitizer, and a second molecule known as the coinitiator that transfers a labile atom – usually a hydrogen atom – to the photosensitizer. In the process, free radicals are formed on the coinitiator, which then initiates polymerization.
This type of photoinitiator system is, perhaps, a bit less elegant than the photocleavage type, not only because it requires two molecules, but also because it typically produces byproducts, which may be undesirable. However, depending on the composition, these may be less expensive than the Norrish I photoinitiators, and the coinitiators also can assist in overcoming the effects of oxygen inhibition. 4 A common example of this type of photoinitiator system contains benzophenone as the photosensitizer along with various amines, alcohols or other species that have labile hydrogen atoms, e.g., N, N-dimethylethanol amine (DMEA) or N-methyldiethanol amine (MDEA).
Technical Questions?
What are your technical questions about polymer science, photopolymerization or other topics concerning the chemistry and technology of UV/EB polymerization? Please submit your questions or comments via email to Dianna Brodine, vice president, editorial for Peterson Media Group, at dianna@petersonmg.com or to me at
b4christmas@gmail.com.
References
- “Professor’s Corner”, UV+EB Technology, Vol. 5, No.1, pg. 14.
- “Professor’s Corner”, UV+EB Technology, Vol. 5, No.3, pg. 13.
- Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/resin. Accessed March 28, 2023.
- Christmas, B. K. & Idacavage, M. J., Photopolymerization: Fundamental Polymer Chemistry & Industrial Applications, DEStech Publications, Inc., 2023, pp. 81-86.
 Byron K. Christmas, Ph.D.
Byron K. Christmas, Ph.D.
Professor of Chemistry, Emeritus
University of Houston-Downtown
b4christmas@gmail.com